Proteins Can Serve All of the Following Functions Except
The edifice blocks of proteins are amino acids, which are small organic molecules that consist of an alpha (fundamental) carbon atom linked to an amino grouping, a carboxyl group, a hydrogen cantlet, and a variable component called a side chain (see below). Within a protein, multiple amino acids are linked together by peptide bonds, thereby forming a long chain. Peptide bonds are formed by a biochemical reaction that extracts a h2o molecule as it joins the amino group of ane amino acid to the carboxyl grouping of a neighboring amino acid. The linear sequence of amino acids inside a protein is considered the chief structure of the protein.
Proteins are built from a set of only twenty amino acids, each of which has a unique side chain. The side chains of amino acids accept dissimilar chemistries. The largest group of amino acids have nonpolar side chains. Several other amino acids accept side chains with positive or negative charges, while others have polar merely uncharged side chains. The chemistry of amino acrid side bondage is critical to protein structure considering these side chains can bond with one another to hold a length of protein in a certain shape or conformation. Charged amino acid side chains tin form ionic bonds, and polar amino acids are capable of forming hydrogen bonds. Hydrophobic side chains interact with each other via weak van der Waals interactions. The vast majority of bonds formed past these side chains are noncovalent. In fact, cysteines are the but amino acids capable of forming covalent bonds, which they do with their item side chains. Because of side chain interactions, the sequence and location of amino acids in a particular protein guides where the bends and folds occur in that protein (Figure 1).
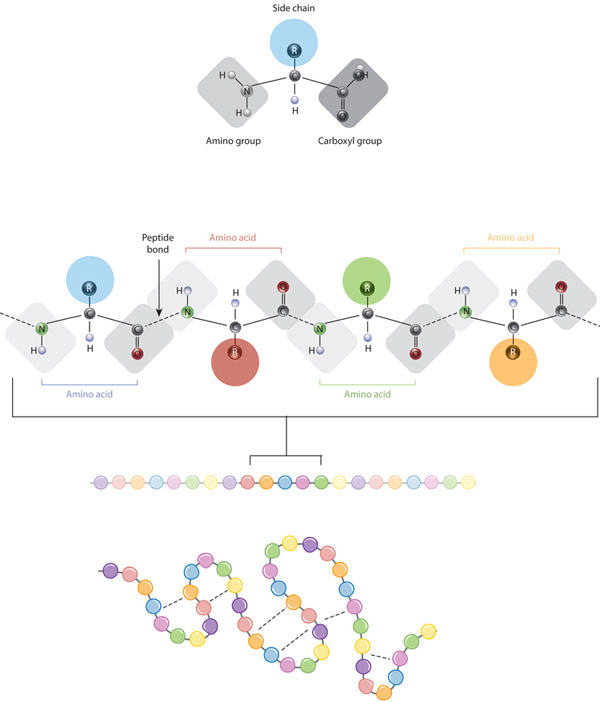
Figure 1: The relationship betwixt amino acid side bondage and poly peptide conformation
The defining feature of an amino acid is its side chain (at top, blue circle; below, all colored circles). When connected together by a serial of peptide bonds, amino acids form a polypeptide, another word for poly peptide. The polypeptide will then fold into a specific conformation depending on the interactions (dashed lines) between its amino acid side chains.
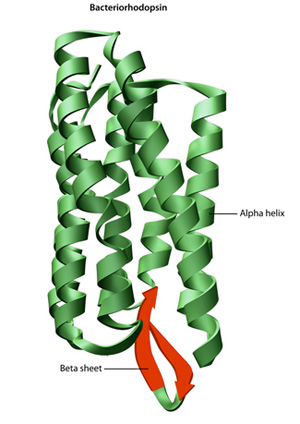
Figure two: The structure of the protein bacteriorhodopsin
Bacteriorhodopsin is a membrane poly peptide in bacteria that acts as a proton pump. Its conformation is essential to its function. The overall structure of the poly peptide includes both alpha helices (green) and beta sheets (red).
The primary structure of a protein — its amino acid sequence — drives the folding and intramolecular bonding of the linear amino acid concatenation, which ultimately determines the protein's unique 3-dimensional shape. Hydrogen bonding between amino groups and carboxyl groups in neighboring regions of the poly peptide chain sometimes causes sure patterns of folding to occur. Known as alpha helices and beta sheets, these stable folding patterns make upwards the secondary structure of a protein. Most proteins contain multiple helices and sheets, in add-on to other less common patterns (Figure two). The ensemble of formations and folds in a single linear chain of amino acids — sometimes chosen a polypeptide — constitutes the tertiary structure of a protein. Finally, the quaternary construction of a protein refers to those macromolecules with multiple polypeptide chains or subunits.
The final shape adopted by a newly synthesized poly peptide is typically the most energetically favorable 1. As proteins fold, they test a variety of conformations before reaching their final form, which is unique and meaty. Folded proteins are stabilized by thousands of noncovalent bonds betwixt amino acids. In add-on, chemical forces between a protein and its firsthand environs contribute to protein shape and stability. For example, the proteins that are dissolved in the cell cytoplasm have hydrophilic (water-loving) chemical groups on their surfaces, whereas their hydrophobic (water-averse) elements tend to be tucked within. In contrast, the proteins that are inserted into the cell membranes brandish some hydrophobic chemical groups on their surface, specifically in those regions where the poly peptide surface is exposed to membrane lipids. It is important to note, nevertheless, that fully folded proteins are not frozen into shape. Rather, the atoms within these proteins remain capable of making small movements.
Even though proteins are considered macromolecules, they are too small to visualize, even with a microscope. And then, scientists must use indirect methods to figure out what they await like and how they are folded. The most common method used to report protein structures is X-ray crystallography. With this method, solid crystals of purified protein are placed in an X-ray beam, and the blueprint of deflected Ten rays is used to predict the positions of the thousands of atoms inside the protein crystal.
Source: https://www.nature.com/scitable/topicpage/protein-structure-14122136/
0 Response to "Proteins Can Serve All of the Following Functions Except"
Post a Comment